Author Name
V. Logronio Ferlyn, and M. Yagos Rosanilio
Journal Name
International Journal of Agronomy and Agricultural Research | IJAAR
Publisher Name
International Network For Natural Sciences | INNSpub
Abstract
Different physiological, morphological, and behavioral adaptations of Pomacea canaliculata aided them in their survival to different adverse environmental conditions. Furthermore, the said adaptations can be very vital in the control and management strategies that can be employed in the areas where their population posed a threat to food security. The study employed an explorative-investigative study design for the gathering of data. Eight hundred seventy-three Golden Apple Snails from different freshwater ecosystems, namely stream, irrigational canal, and rice field were collected, cleaned, and examined. To elucidate the different adaptations of the GAS to the various ecosystems, their shell characteristics were observed, recorded, and examined. Consequently, this study found out that those shells from snails sampled in streams had bigger length, width, width of the aperture, a higher number of bands, and whorls when compared to those shells from irrigational canals and rice fields. Moreover, there was a negative correlation between pH and dissolved oxygen to the height, width, and width of the aperture. There was also a significant correlation between the temperature and width, weight, and the number of bands. It was concluded that to control and manage the population of the GAS the area should have less palatable food sources and less anthropogenic activities so that environmental parameters like high pH, lower temperature, and higher dissolved oxygen can be achieved.
Introduction
The physiological, morphological, and behavioral adaptation of Pomacea canaliculata to various environmental conditions made them thrive and be one of the major pests in freshwater wetlands particularly affecting ricefields and other economically important agricultural areas. They had been known for their adaptive plasticity (Estebenet & Martin, 2002) and highly generalist and voracious macrophytophagous feeding nature (Morrison, et al., 2016).
P. canaliculata, or commonly known as golden apple snail, is one of the freshwater snails that underwent series of adaptations to thrive in any given freshwater environment. Hence, the golden apple snail can tolerate harsh environmental conditions such as low levels of salinity, lower temperature (Seuffert, et al., 2013), low pH levels, metal and pollutant contamination, parasite infestations, period of drought (Silverwood, 2011), and low food availability (Tamburi & Martin, 2016). However, its survival in these adverse environmental conditions is highly dependent on the important physiological, morphological, and behavioral adaptations (Chukwuka, et al., 2014) of these snails to their environment. Furthermore, according to Relyea (2002), as cited by Madjos, et al., in 2015 that P. canaliculata can respond to the changes in the environment by producing alternative phenotypes as an adaptive strategy.
Consequently, there had been different functional parts of the snails that played an important factor in their survival especially in maintaining their homeostasis. One of those parts is the shell. Salient features of the snails’ shells that ensured the snails’ survival against adverse environmental conditions, predator cues, and various anthropogenic-related activities were: shell periostracum, shell chirality, shell color, shell shape, shell size and weight, and operculum shape and weight.
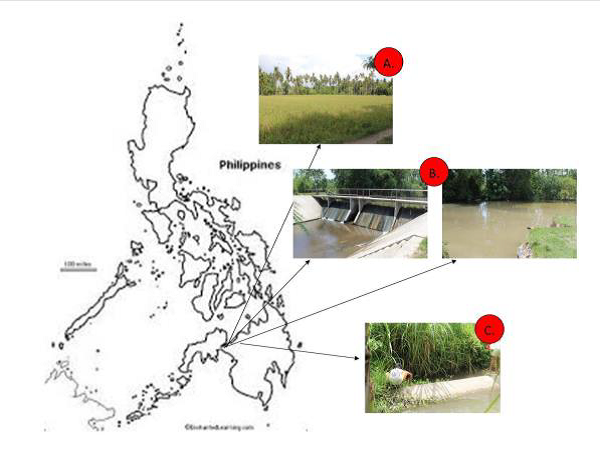
However, these characteristics could also be a potential target for control measures. A better understanding of the important characteristics of the species that helped them survive various environmental stresses should be understood by people implementing management and control programs. Henceforth, this paper was conducted to provide basic information on the adaptation of the snails based on the characteristics of their shells, so that proper management and control measures can be crafted to make them better suited in the area. The paper looked into three different ecosystems which have important economic value where the presence and manifestation of golden apple snails were seen to be of great concern. Get the full articles by following the link Int. J. Agron. Agri. Res.20(1), 24-32, January 2022
Reference
Boettiger A, Ermentrout B, Oster G. 2009. The neural origins of shell structure and pattern in aquatic molluscs. PNAS 106(16), 6837-6842.
Cazzaniga N. 1987. Pomacea canaliculate (Lamrk, 19801) en Catamarca (Argentina) y un comentario sobre Ampullariacatamarcensis Sowerby, 1874 (Gastopoda, AMpullaridae). Iheringia, Serie Zoologia 66, 43-68.
Chukwuka CO, Ejere VC, Asogwa CN, Nnamonu EI, Okeke OC, Odii EI, Ugwu GC, Okanya LC, Levi CA. 2014. Eco-physiological adaptation of the land snail Achatina achatina (Gastropoda: Pulmonata) in tropical agro-ecosystem. The Journal of Basic and Applied Zoology 67, 48-57
Comfort A. 1950. The Pigmentation of Molluscan Shells. 285-300.
Ermentrout B, Campbell J, Oster G. 1986. A Model for Shell Patterns Based on Neural Activity. The Velger 28(4), 369-388.
Estebenet A, Martin P. 2002. Pomacea canaliculata (Gastropoda: Ampullariidae): Life-history and Traits and their Plasticity. Biocell 26(1), 83-89.
Estebenet A, Martin P. 2003. Shell Interpopulation Variation and its Origin in Pomacea canaliculata (Gastropoda: Ampullariidae) from Southern Pampas, Argentina. J. Moll. Stud 60, 301-310.
Estebenet A, Martin P, Burela S. 2006. Conchological variation in Pomacea canaliculate and other South American Ampullariidae (Caenogastropoda, Architaenioglossa). Biocell 30(2), 329-335.
Fink P, Von Elert E. 2006. Physiological responses to stoichiometric constraints nutrient limitation and compensatory feeding in a freshwater snail. Oikos 115, 484-494.
Galan G, Porquis H, Bulasa M. 2015. Shell band pattern of Golden Apple Snail (Pomacea canaliculate, Lamark) in Selected Aquatic Habitats. International Journal of Environmental Science and Development 6(8), 625-628.
Glass N, Darby P. 2009. The effect of calcium and pH on Florida apple snail, Pomacea paludosa (Gastropoda: Ampullariidae), shell growth and crush weight. Aquatic Ecology 43(4), 1085-1093.
Gould S. 1966. J Paleontol 42, 81-98
Gupta A, Pereira C, Soukup J. 2017. Effects of thermal stress on growth and mortality of juvenile Crepidula fornicate in New England. Marine Physiology and Climate Change 2017, 1-9.
Kalinda C, Chimbari M, Mukaratirwa S. 2017. Effect of temperature on the Bulinus globosus – Schistosoma haematobium system. Infectious Diseases of Poverty 2017(6), 57.
Kemp P, Bertness M. 1984. Snail shape and growth rates: Evidence for plastic shell allometry in Littorina littorea. Proc. Natl. Acad. Sci, USA. 81, 811-813
Madjos G, Anies O. 2016. Morphometrics approaches to studying phenotypic plasticity in Pomacea canaliculate (Golden apple snail). International Journal of Advanced and Applied Sciences 3(4), 50-56.
Madjos G, Demetillo M, Baguio M, Torres M. 2015. Phenotypic variation in populations of Pomacea canaliculata (Golden Apple Snail): a case of agroecotypes?. Advances in Environmental Sciences – International Journal of the Bioflux Society 7(3), 432-441.
Mahilum J, Demayo C. 2014. Sexual Dimorphism on Shell Shape of Pomacea canaliculata Lamark Thriving in Lakes using the Geometric Morphometric Approach. International Journal of Bioscience, Biochemistry, and Bioinformatics 4(4), 284-289
Marshall D, Santos J, Leung K, Chak W. 2008. Correlations between gastropod shell dissolution and water chemical properties in a tropical estuary. Marine Environmental Research 66(4), 422.
Memon U, Balich W, Tunio G, Burdi G, Korai Al, Pirzada A. 2011. Food, feeding, and growth of Golden Apple Snail Pomacea canaliculate, Lamark (Gastropoda: Ampullariidae). Sindh University Research Journal (Science Series) 43(1), 25-28.
Minton R, Lewis E, Netherland B, Hayes D. 2008. Large Differences over Small Distances: Plasticity in the Shells of Elimia potosiensis (Gastropoda: Pleuroceridae). International Journal of Biology 3(1), 23-32c.
Morrison W, Hay Mark. 2016. Feeding and growth of native and exotic apple snails (Ampullariidae): Invasive eat more and grow more. Accessed at http://www.lib.noaa.gov/about/news /Morrison_06302010.pdf
Orr J, Fabry V, Aumont O, Bopp L, Doney S, Feely R, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key R, Linsay K, Maier-Reimer E, Matear R, Monfray F, Mouchet A, Najjar R, Plattenr G, Rodgers K, Sabine C, Sarmiento J, Schlietzer R, Slater R, Totterdel I, Weirig M, Yamanaka Y, Yool A. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681-686.
Samsi A, Karim S. 2019. The relationship between the length and weight of snail Nerita lineata Gmelin1791 on environmental factors in the mangrove ecosystem. Journal of Physics: Conference Series 1341(2019).
Seuffert M, Martin P. 2013. Juvenile growth and survival of the apple snail Pomacea canaliculata (Casnogastropoda: Ampullariidae) reared at different constant temperatures. SpringerPlus. 2-312
Silverwood K. 2011. Apple Snail Risk Analysis for Arizona. Accessed at http://www.azgfd.gov/h_f/ documents/AIS-AppleSnailRisk.pdf
Sokolova I, Berger V. 2000. Physiological variation related to shell color polymorphism in White Sea Littorina saxatillis. Journal of Experimental Marine Biology and Ecology 245(2000), 1-23
Tamburi N, Martin P. 2016. Effects of absolute fasting on reproduction and survival of the invasive apple snail Pomacea canaliculata in its native range. Current Zoology 62(4), 369-375.
Tamburi N, Seuffert M, Martin P. 2018. Temperature-induced plasticity in morphology and relative shell weight in the invasive apple snail Pomacea canaliculate. Journal of Thermal Biology 74(4).
Yam R, Fan Y, Wang T. 2016. Importance of Macrophyte Quality in Determining Life-History Traits of the Apple Snails Pomacea canaliculata: Implications for Bottom-up Management of an Invasive Herbivorous Pest in Constructed Wetlands. International Journal of Environmental Research and Public Health 13, 248.